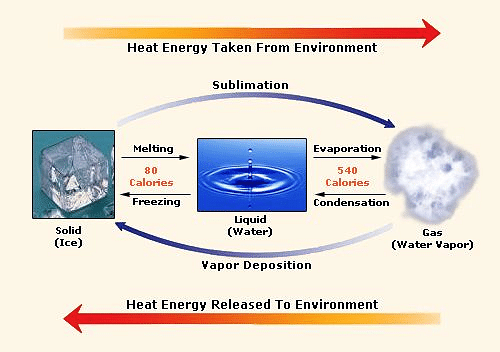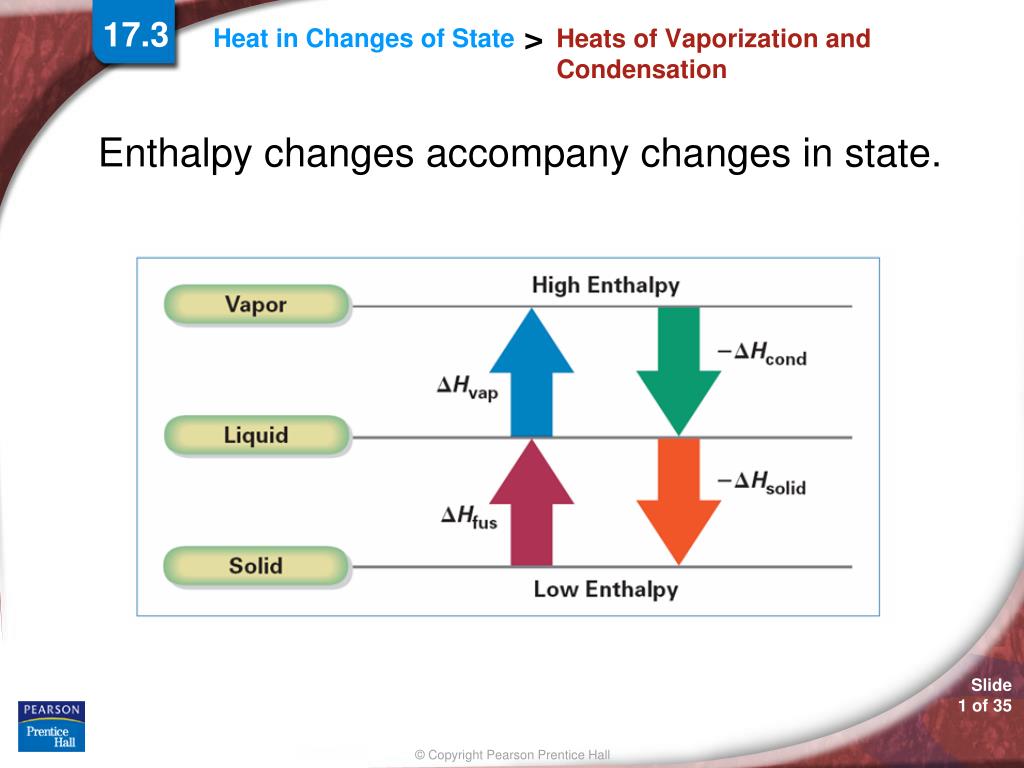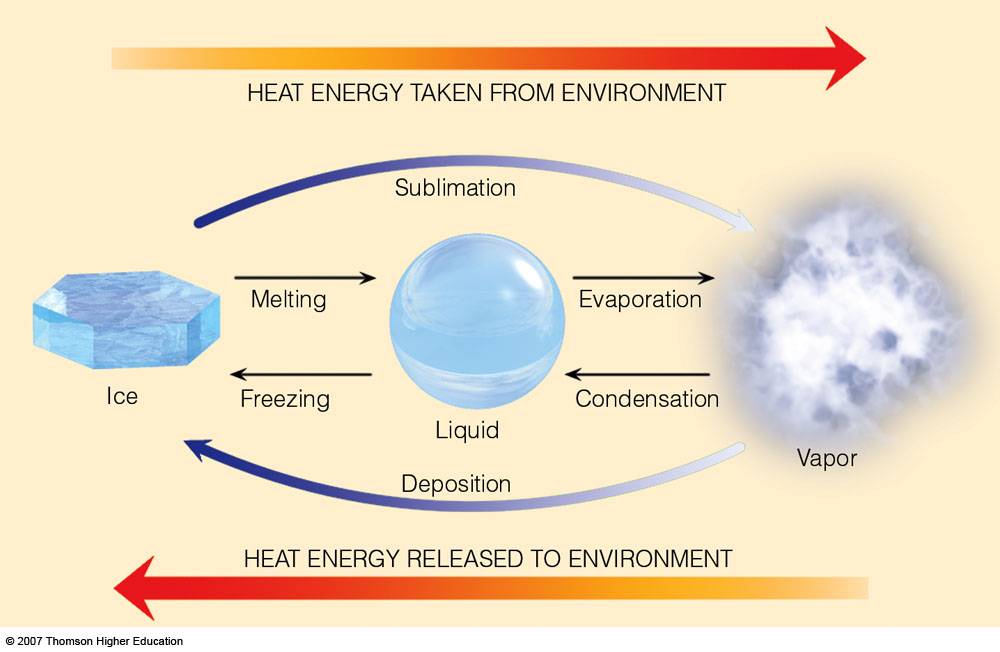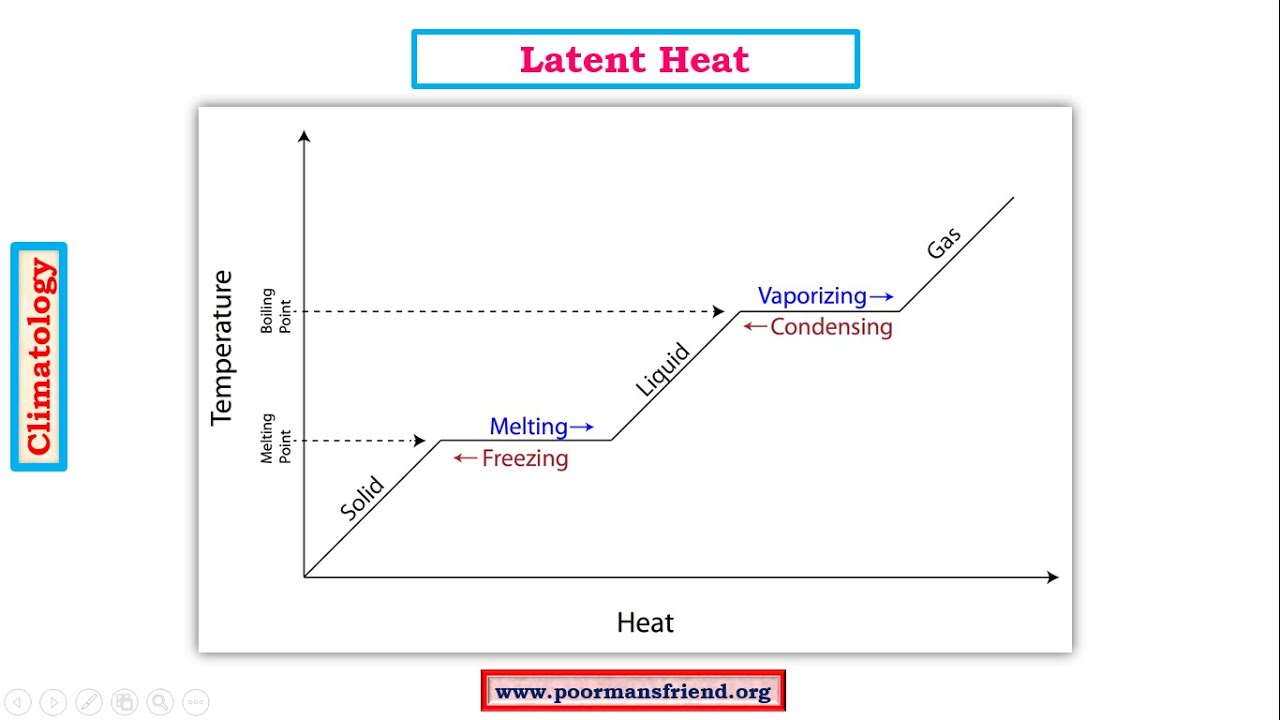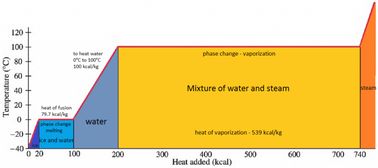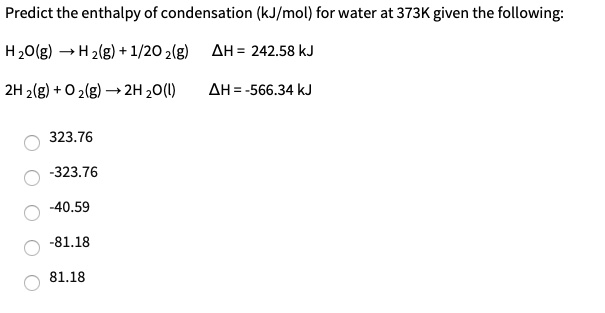
SOLVED: Predict the enthalpy of condensation (kJ/mol) for water at 373K given the following: HzOlg) Hzlg)- 1/20 2(g) AH = 242.58 kJ 2H 2(g) 0 2lg) 2H 20() AH =-566.34kJ 323.76 323.76 40.59 81.18 81.18

Given, `H_(2)(g)+Br_(2)(g)to2HBr(g),DeltaH_(1)^(@)` and standard enthalpy of condensation of - YouTube

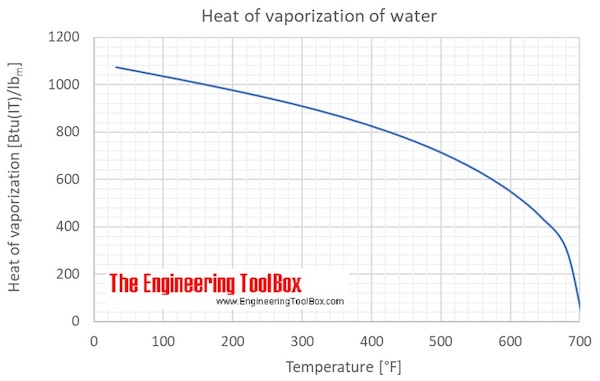
enthalpy - What is heat of vaporization? How can it be used at temperature as low as 25 °C? - Chemistry Stack Exchange

Given, H2(g) + Br2(g)→ 2HBr(g), Δ H^∘1 and standard enthalpy of condensation of bromine is Δ H^∘2 , standard enthalpy of formation of HBr at 25^∘C is:

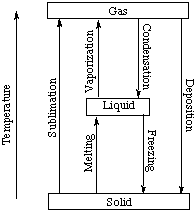
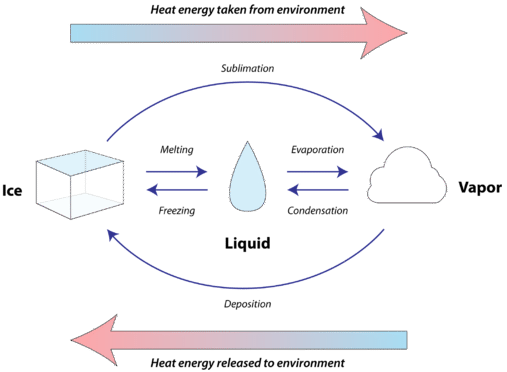
Premium Vector | Phase changes vector illustration. labeled matter scheme with enthalpy system. diagram with plasma, solid, gas and liquid transformation. ionization, condensation, sublimation and vaporization example